Established in March 2018, Korea Bio-health Innovation & Startup Center (hereafter, ‘K-BIC) bridges the gap between technology R&D and commercialization, particularly supporting early startups in the bio-health industry. In a conversation with Seoung-jun Park, the director of the center, how the center functions to support bio-health startups will be given.

K-BIC provides full-cycle support for the startups in the biohealth industry. About 600 startups are under the support of K-BIC. Mr. Park explains that around 800 enterprises with patent technologies come up in the bio and health industry every year, and from 200 to 300 of them are annually supported by the center. Unfortunately, around 20% of startups fail during the first year of operations. Understanding the key stages of the business life cycle is crucial to ensuring that small business avoids that fate. For small and medium-sized enterprises, business growth is close to their legacies so they strive to keep them relevant in the long-run. Each business life cycle stage comes with unique and strategic management. To plan strategic management, startups would need some help from professionals to identify at which stage of the business life cycle the enterprise is, because that will define the direction of your operations and inform your company’s strategic planning.
According to Mr. Park, startups at the very beginning stage that the company forms an idea for commercialization can garner advice and opinion from professional tech scouts in the center. They can give the startups tailored consultation about if the technologies are worth developing into an actual business. Particularly, Project Managers (PMs) in K-BIC offer tailored and proactive management for early market advancement of promising technologies. PMs advise companies to turn existing intellectual property into products, finding the right markets, messaging, and features. Mr. Park explains that it is a unique system compared to other startup centers and PMs try their best to help startups as if they are pacemakers in a marathon. Moreover, by utilizing connections with other organizations for commercializations and around 1,000 professional mentors, startups can be given an evaluation of ideas’ business feasibility. It is very crucial help for them since most startups struggle due to a lack of demand for their products or services.
Obtaining a proper patent is also essential for startups to create a business plan. The patent strategy consulting will be given to nurtured startups in K-BIC for securing intellectual property rights and practical application. The given strategy is tailored to different patents from different countries. Furthermore, the center supports the startups’ clinical tests within networks among industry, academia, research institutes, and hospitals. K-BIC also holds a conference named ‘Bio Korea’ and operates an online business networking platform named ‘Bio Agora’, which leads startups to reach out to investors and communicate or share technologies and businesses.
The director Park said that all staff members in the center help startups optimize market targeting and positioning for success. To uncover hidden market opportunities, the K-BIC will not stop until the end.
Here below are four excellent startups which are being supported by the K-BIC.
BIORCHESTRA to conquer Alzheimer’s dementia and incurable diseases
BIORCHESTRA is a venture company mainly focusing on research and development of therapeutics based on ribonucleic acid (RNA).
We plan to take the lead on improving the health of humankind with the core technology of developing RNA based new medicines by developing therapeutics for incurable diseases through a paradigm shift from alleviation of symptoms to fundamental treatments.

BIORCHESTRA approach to Alzheimer’s disease is quite different from conventional approach. Most of conventional pharmaceutical companies are focusing on reducing pathological protein such as Aβ or tau. However, BIORCHESTRA has taken a different approach of targeting pathological microRNA that are upstream to various AD-related pathological processes. For instance, by upregulating CD36, SIRT1 and PGC-1α, we have been able to improve Aβ clearance, neuroinflammation, neuro-restoration as well as cognitive impairment in a comprehensive and simultaneous manner. This upstream regulation with the help of our anti-sense oligonucleotide aims to reverse the epigenetic changes in the AD brain induced by the overexpression of our target microRNA-485-3p, which we have proven to induce Aβ/tau production, cognitive impairment and a reduction in synaptic plasticity. Furthermore, through this upstream regulation, we have also been able to successfully activate ADAM10 and downregulate BACE1 activity, enzymes that are involved in the processing of amyloid precursor proteins, thereby reducing Aβ production.
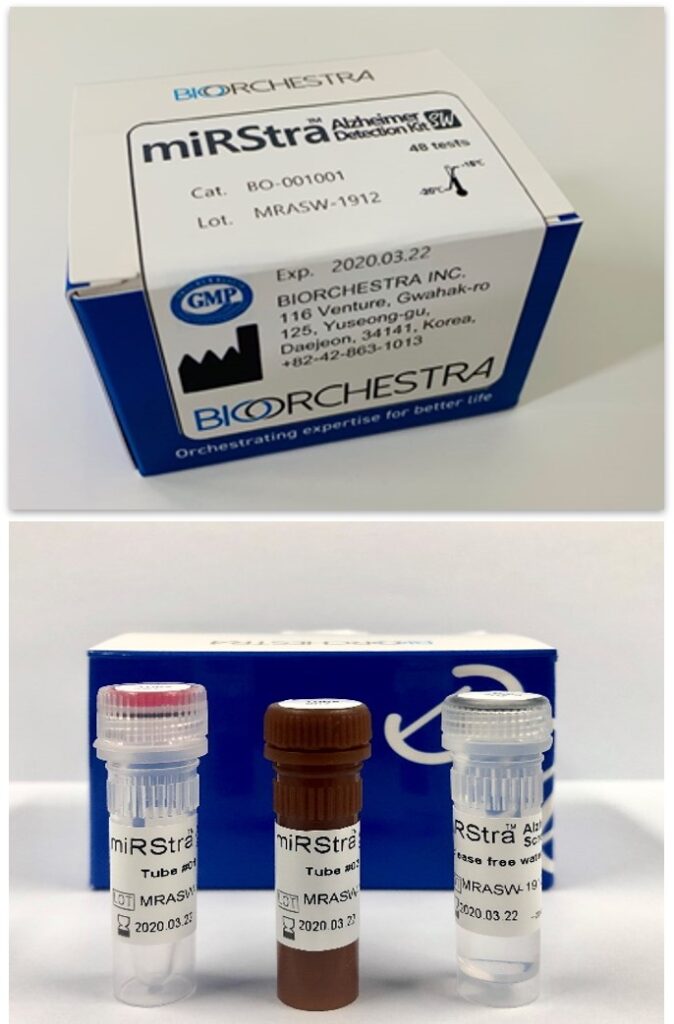
We developed and applied a brain-specific drug delivery system using proprietary technology to safely deliver Alzheimer’s disease drug candidates (ASO) to brain cells through intravenous injection. The transmittance efficiency is about 7% in rodent, which is the highest level compared to the results of other research groups known to date.
Recently BIORCHESTRA is performing feasibility study with some global pharmaceutical companies to license out this brain target drug delivery system. One global pharmaceutical company is conducting technical due diligence for licensing deal.
BIORCHESTRA got award from Johnson and Johnson in neuroscience. Branden Ryu said “We are pleased that scientist in Johnson and Johnson reviewed and awarded on Biorchestra science”
Our goal is to switch the therapeutic paradigm from treatment of symptoms to fundamental cure of disease. As we are progressing clinical development, we are seeking a global partner for R&D collaboration and drug commercialization.
Biot Korea with innovative solution for maximizing treatment
Biot Korea (CEO Yeong-jun Chang) is a medical robot startup established in June 2017. It is developing the world’s first medical robot in the field of diagnosis and treatment, and also developing commercialized products by transferring new medical device source technology from Korea Micro Medical Robot Research Institute and Korea Institute of Machinery and others. Young researchers majoring in material, mechanical, and systems engineering are co-hosted by hospitals and research institutions.

In the field of treatment, the micro-medical robot platform, “Stem Cell Navigator,” which maximizes the efficacy of treatment by inducing and depositing various treatments to patients in the body, and in the field of diagnosis, “non-face-to-face respiratory virus sample collecting robots” to prevent secondary infections and sustainable quarantine conditions. Stem cell navigator technology is a medical device platform technology that can physically deliver treatments to osteoarthritis patients with minimal invasive drug active delivery and deposition systems. When a treatment is injected into an affected area of osteoarthritis, the treatment spreads to the live fluid in the joint cavity, halving the effects of the treatment, such as cartilage regeneration. Stem cell navigators are minimally invasive for physical transfer and deposition within the treatment’s affected area. It is expected that osteoarthritis can be suppressed in patients with early and mid-term osteoarthritis.
Biot Korea conducted a heavy animal potency evaluation on rabbits and proved that osteoarthritis is more effective than simple self-stem cell injections when treating osteoarthritis self-stem cells through stem cell navigators. It was published in Science Robotics in last January. Non-face-to-face sample collection robots were developed to reduce fatigue of medical staff and prevent secondary infections. It is a miniaturized six-degree-based robot system that allows the patient’s respiratory samples to be collected without facing medical staff.
1drop Inc. with convenient healthcare solution
1drip Inc. is a leading mobile healthcare company through technological innovation. It is a company that specializes in providing smartphone-based solutions for managing chronic diseases with a drop of bloods, and establishes and develops traditional molecular diagnosis products. More than just this is the case, recently, company is launching for molecular diagnosis platform to diagnosis COVID-19 as field-type products for the first time in Korea.
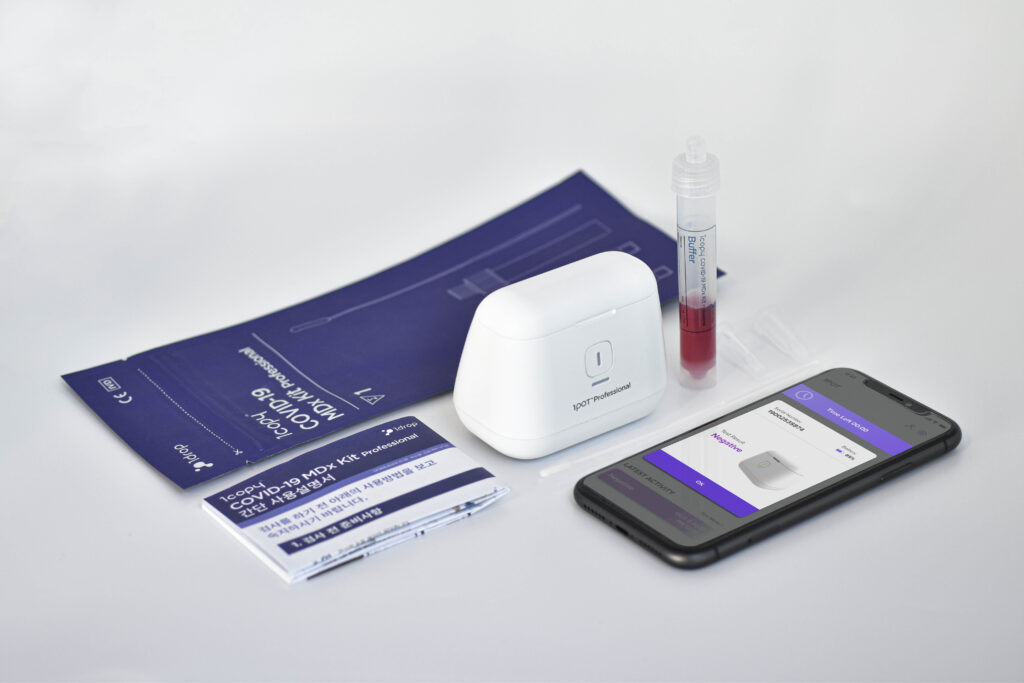
As you know, the coronavirus has put the world at risk for secondary infections, especially the delta virus. Developed countries are already working hard on quarantine and diagnostic tests to prevent the spread of transmission by developing treatments. In this situation, we will be very happy and interested in convenience if we can take care of our health through a drop of body fluid with the closest mobile in our daily lives. In particular, South Korea is known as a model country in the quarantine system and has actively implemented the development and response of the initial diagnostic kit. In other words, we can see that it is sponsoring the management of the Korean health industry and the research and development of companies.
Even before the coronavirus outbreak, several large companies, especially IT companies, had a lot of interest and investment in digital health. This may be because the sensitive aspects of healthcare and healthcare are also challenges that need to be addressed, which require professional and accurate diagnosis. As the world is already building big data with the development of mobile communication data, we should pay attention to the company’s move because it is clear that collective infections, personal health and quality of life will be improved if many scaled technologies are also developed and utilized in healthcare.
G.T.I with manufacturing quality management systems
G.T.I. is a company that develops and builds software for manufacturing quality management systems for the safety and accuracy of pharmaceutical and bio information.
Specifically, the Cell Vestige is software for microbial analysis and diagnostic testing performed on pharmaceutical, bio-industrial sites.

Cell Vestige can be used in a variety of industries. Typical examples include microbial testing in pharmaceutical, in-vitro diagnostic, medical equipment, cosmetics and food industries that require GMP certification can be used in hospital clinical microbial tests and microbial laboratories.
Cell Vestige is eight times more efficient than the traditional Microbiological Test Method, which provides a business automation process that can reduce the time taken to 45 minutes from about 370 minutes of experimental data.
As you know, even small differences in ingredients and materials in the pharmaceutical bio-field can cause significant damage to the human body, but they also need to be managed for transparency in managing raw data and in manufacturing development. And it will be helpful to improve the accuracy and quality of test analysis by combining the scaled big data with artificial intelligence to establish a system that can be checked from time to time. All pharmaceutical bio-companies are required to inform and comply with standard processes in advance.
They are submitted in lieu of photographs or records manipulated in the process of reporting without actually attaching the tested data. In some cases, experimental data themselves are not stored. These acts also belong to illegal manufacturing. We want to lead a healthy life, but we have no choice but to rely on expressed and informed facts about the efficacy of pharmaceutical companies and bio-companies. Professional knowledge and medical findings are obtained by professionals through prescription and medical treatment. If all of this is transparently supplied to us, it will certainly help us live a healthy life.
SAM KIM
KAYLA HONG
Asia Journal



